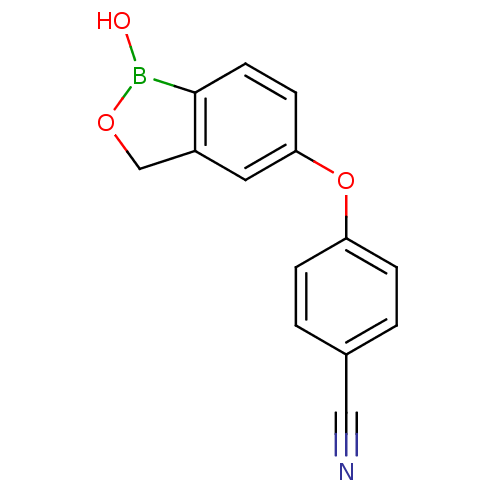
BDBM50277665 4-(1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-5-yloxy)benzonitrile::5-(4-cyanophenoxy)-2,3-dihydro-1-hydroxy-2,1-benzoxaborole::AN-2728::CHEMBL484785::Crisaborole::Eucrisa
SMILES OB1OCc2cc(Oc3ccc(cc3)C#N)ccc12
InChI Key InChIKey=USZAGAREISWJDP-UHFFFAOYSA-N
Data 22 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50277665
Found 5 hits for monomerid = 50277665
Affinity DataIC50: 110nMAssay Description:Inhibition of human PDE4 catalytic domain expressed in Escherichia coliMore data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Inhibition of PDE4B1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 55nMAssay Description:Inhibition of PDE4B2 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 57nMAssay Description:Inhibition of PDE4B (unknown origin) assessed as hydrolysis of [3H]-cGMP into [3H]-GMP incubated for 30 mins by scintillation of proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+4nMAssay Description:Inhibition of PDE4B (unknown origin)More data for this Ligand-Target Pair